In situ spectroscopic characterization of a solution-phase X-type ligand exchange at colloidal lead sulphide quantum dot surfaces†
Abstract
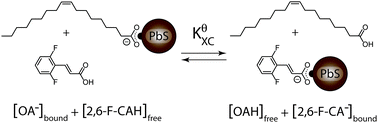
We employed quantitative NMR spectroscopy and spectrophotometric absorbance titration to study a quantum dot X-type ligand exchange reaction. We find that the exchange is highly cooperative, where at low extents of exchange the change in free energy of the reaction, ΔGXC, is ∼11 kJ mol−1 while at higher extents of exchange ΔGXC saturates to ∼−4 kJ mol−1. A modified Fowler binding isotherm is developed to describe the reaction.
- This article is part of the themed collections: Celebrating our 2019 Prize and Award winners and Frontiers in Nanocrystal Surface Chemistry


 Please wait while we load your content...
Please wait while we load your content...