Palladium-catalyzed direct intermolecular silylation of remote unactivated C(sp3)–H bonds†
Abstract
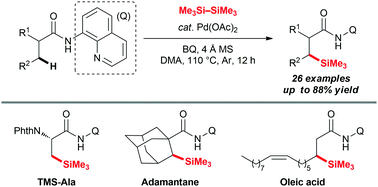
An efficient and convenient method has been developed to achieve direct silylation of unactivated remote primary or secondary C(sp3)–H bonds to form C–Si bonds with hexamethyldisilane (HMDS). This method highlights the emerging strategy to transform unactivated methyl or methylene into versatile functional groups in organic synthesis and provides a new method to construct functionalized C–Si bonds for synthetic chemistry.

 Please wait while we load your content...
Please wait while we load your content...