Tuning the structure of 1,3,5-benzene tricarboxamide self-assemblies through stereochemistry†
Abstract
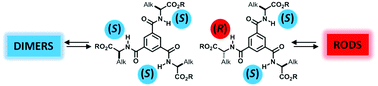
A heterochiral 1,3,5-benzene tricarboxamide (BTA) monomer, derived from valine dodecyl ester, forms long rods in cyclohexane whilst its homochiral analogue assembles into dimers only at the same concentration. This highly original assembly behaviour is related to the destabilization of the dimeric structure containing the two heterochiral monomers as corroborated by a combined experimental and computational study.
- This article is part of the themed collection: Chirality at the nanoscale


 Please wait while we load your content...
Please wait while we load your content...