Stability of organic compounds on the oxygen-evolving center of photosystem II and manganese oxide water oxidation catalysts†
Abstract
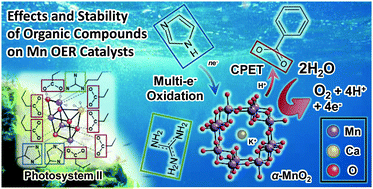
Surrounding organic residues are indispensable for the high water oxidation activity of the Mn4 cluster in photosystem II. Here, the stability of organic compounds on synthetic Mn oxides was analyzed using inlet electrochemical mass spectroscopy. Carboxyl groups, which are the most abundant residues around the Mn4 cluster, were found to stably facilitate the oxygen evolution reaction.

 Please wait while we load your content...
Please wait while we load your content...