Chloromethyl-triazole: a new motif for site-selective pseudo-acylation of proteins†
Abstract
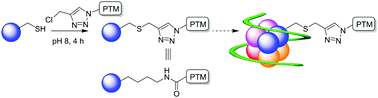
Rapid, site-selective modification of cysteine residues with chloromethyl-triazole derivatives generates pseudo-acyl sLys motifs, mimicking important post-translational modifications. Near-native biotinylation of peptide and protein substrates is shown to be site-selective and modified histone H4 retains functional activity.


 Please wait while we load your content...
Please wait while we load your content...