Crystal engineering of a zwitterionic drug to neutral cocrystals: a general solution for floxacins†
Abstract
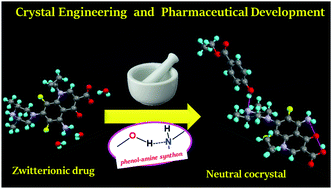
The transformation of zwitterionic Sparfloxacin (SPX) to the neutral form is achieved by cocrystallization. Neutral forms of drugs are important for higher membrane permeability, while zwitterions are more soluble in water. The twin advantages of higher solubility/dissolution rate and good stability of neutral SPX are achieved in a molecular cocrystal compared to its zwitterionic SPX hydrate. The amine-phenol supramolecular synthon drives cocrystal formation, with the paraben ester acting as a “proton migrator” for the ionic to neutral transformation.

 Please wait while we load your content...
Please wait while we load your content...