Stable bromoallene oxides†
Abstract
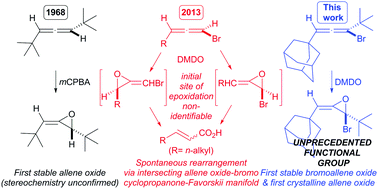
The first stable bromoallene oxides were obtained by the DMDO epoxidation of 1-bromo-1,3-di-tert-alkylallenes, producing the first crystalline allene oxide of any kind. The epoxidations are regioselective for the bromine-bearing Δ1,2 alkene, and also face selective producing single diastereomer E-olefin products.


 Please wait while we load your content...
Please wait while we load your content...