Metal-free C–H sulfonamidation of pyrroles by visible light photoredox catalysis†
Abstract
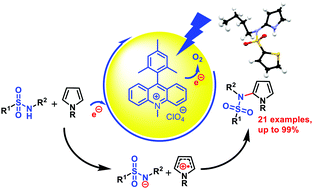
We report a one-step procedure for the preparation of N-(2-pyrrole)-sulfonamides from sulfonamides and pyrroles. The reaction uses visible light, an acridinium dye as photocatalyst and oxygen as the terminal oxidant for the oxidative C–N bond formation; structures of several reaction products were confirmed by X-ray structure analysis. The reaction is selective for pyrroles, due to the available oxidation power of the photocatalyst and the required stability of the carbocation intermediate under the reaction conditions.


 Please wait while we load your content...
Please wait while we load your content...