Helix–helix inversion of an optically-inactive π-conjugated foldamer triggered by concentration changes of a single enantiomeric guest leading to a change in the helical stability†
Abstract
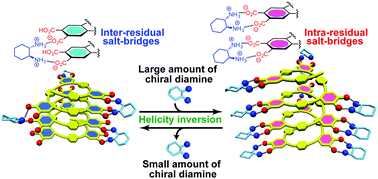
A preferred-handed helicity induced in an optically-inactive poly(phenyleneethynylene)-based foldamer bearing carboxylic acid pendants upon complexation with a single enantiomeric diamine was subsequently inverted into the opposite helix upon further addition of the diamine, accompanied by a remarkable change in the stability of the helices.
- This article is part of the themed collection: Chirality at the nanoscale

 Please wait while we load your content...
Please wait while we load your content...