A lanthanide complex for metal encapsulations and anion exchanges†
Abstract
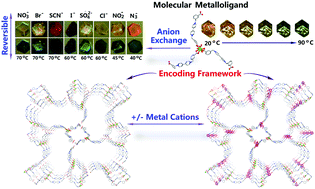
A cationic lanthanide metalloligand with 3 dangling carboxylate groups on its periphery co-assembles with nitrate into a porous thermochromic solid responsive to both external cations and anions, owing to the presence of exchangeable NO3− as well as cation cavities arising from cooperative orientation of free carboxylate groups. An especially interesting feature is the structural memory effect during crystallization exhibited by the metalloligand, even after dissolution and binding to secondary cations (Cu2+, Cd2+…). Moreover, the porous solid can undergo ion-exchange with various anions, leading to tunable thermochromic temperature and color range.


 Please wait while we load your content...
Please wait while we load your content...