Gold-catalyzed cyclization of 1,6-diynyl dithioacetals via 1,7-carbene transfer and aromatic C–H functionalization†
Abstract
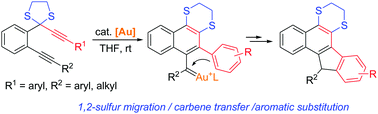
A gold-catalyzed cyclization of 1,6-diynyl dithioacetals has been developed, which provides an attractive route to diverse-substituted benzo[a]fluorene derivatives. The reaction is initiated through 1,2-sulfur migration of a propargyl dithioacetal moiety to generate a vinyl gold carbene, which is followed by carbene transfer to the remaining alkyne and aromatic substitution to furnish the fused products in generally good to high yields.

 Please wait while we load your content...
Please wait while we load your content...