Azaindole synthesis through dual activation catalysis with N-heterocyclic carbenes†
Abstract
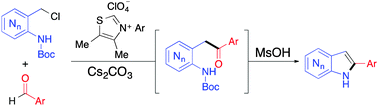
A convergent, transition-metal-free synthesis of 2-aryl-azaindoles has been developed. The interception of a reactive aza-ortho-azaquinone methide intermediate by an acyl anion equivalent generated through carbene catalysis provides high yields, a wide substrate scope, and the synthesis of previously inaccessible azaindoles.

 Please wait while we load your content...
Please wait while we load your content...