A biosynthesis-inspired approach to over twenty diverse natural product-like scaffolds†
Abstract
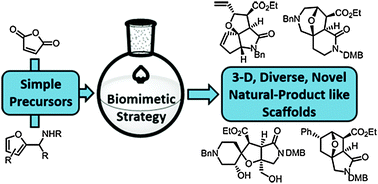
A synthetic approach to diverse scaffolds was developed that was inspired by diterpene biosynthesis. Initial scaffolds, generated using Diels–Alder reactions of furyl-functionalised amines, were transformed into alternative scaffolds using cleavage, ring expansion, annulation and rearrangement reactions. In total, 25 diverse scaffolds were prepared that were shown to have high natural product-likeness.


 Please wait while we load your content...
Please wait while we load your content...