Exploring structural features of folded peptide architectures in the construction of nanomaterials†
Abstract
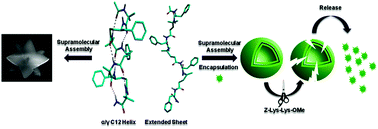
We are reporting the influence of foldamer structures on their self-assembled architectures. In a sharp contrast to the ordered α,γ-hybrid 12-helix obtained from 1 : 1 alternating Aib and γ-Phe, the α,γ-hybrid peptides constituted with α-Phe and 4,4-dimethyl γ-amino acid (Aic) displayed the extended sheet type of conformations in solution and spontaneously self-assembled into thermally and proteolytically stable capsules. In contrast, the conformationally ordered 12-helix self-assembled into a three-dimensional supramolecular polyhedron.

 Please wait while we load your content...
Please wait while we load your content...