The different roles of a cationic gold(i) complex in catalysing hydroarylation of alkynes and alkenes with a heterocycle†
Abstract
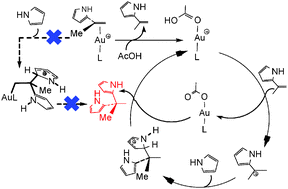
The mechanism of twofold hydroarylation of terminal alkynes with pyrrole catalyzed by a cationic gold(I) complex was investigated using DFT. It was found that while both the hydroarylation reactions proceed via a Friedel–Crafts-type mechanism, the first hydroarylation is directly promoted by gold(I) but the second hydroarylation by a proton released through interaction of the alkene product with gold-bound acidic organic species such as acetic acid and terminal alkynes.

 Please wait while we load your content...
Please wait while we load your content...