Abstract
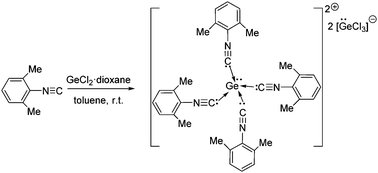
Herein, we introduce isocyanide as a ligand in main group chemistry and describe the facile isolation of a Ge(II) dication. The reaction of 2,6-dimethylphenylisocyanide with GeCl2 leads to the formation of a Ge(II) dication with two [GeCl3]− molecules as counter anions. The dicationic Ge(II) center is bound to four isocyanide ligands and also holds a lone pair of electrons. DFT calculations reveal that the dication is stabilized only by σ-donation from the four isocyanide ligands. Natural population analysis gives a charge of +0.74 on the Ge(II) center, indicating that the positive charge is shared by the isocyanide substituents.

 Please wait while we load your content...
Please wait while we load your content...