Enantioselective total synthesis of (+)-Lingzhiol via tandem semipinacol rearrangement/Friedel–Crafts type cyclization†
Abstract
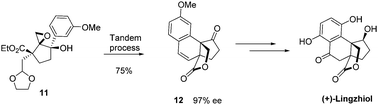
Enantioselective total synthesis of (+)-Lingzhiol has been achieved. It is the first example of in tandem semipinacol rearrangement reactions, the migrated aryl group further reacting with the carbonyl oxonium electrophile to furnish a polycyclic skeleton. Our synthesis involves 13 steps and proceeds in 6% overall yield.

 Please wait while we load your content...
Please wait while we load your content...