A single-step acid catalyzed reaction for rapid assembly of NH-1,2,3-triazoles†
Abstract
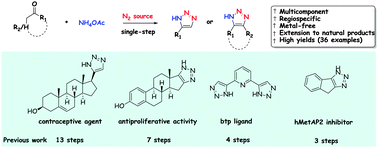
NH-1,2,3-Triazole moieties are a part of the design of various biologically active compounds, pharmaceutical agents and functional materials. Unfortunately, the applications of this heterocycle are still underexplored due to the lack of a general synthetic protocol. Here we outline a novel, general and facile metal-free pathway that enables the direct synthesis of these heterocycles by combining readily accessible and abundant precursors such as enolizable ketones and NH4OAc with high levels of regioselectivity via an organocascade process. The developed chemistry has been successfully applied to the synthesis of several structurally diverse products, pharmaceutical agents and supramolecular receptors.

 Please wait while we load your content...
Please wait while we load your content...