Catalyst-controlled switch of regioselectivity in the asymmetric allylic alkylation of oxazolones with MBHCs†
Abstract
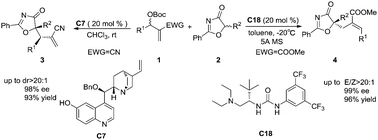
A catalyst-controlled switch of regioselectivity in asymmetric allylic alkylation of oxazolones with MBHCs was described. The SN2′–SN2′ reaction catalysed by a quinine-derived base produced γ-selective secondary allylic oxazolone derivatives, whereas the addition–elimination reaction catalysed by an amino acid-derived bifunctional urea catalyst provided β-selective primary adducts.

 Please wait while we load your content...
Please wait while we load your content...