Silver-mediated direct trifluoromethoxylation of α-diazo esters via the −OCF3 anion†
Abstract
Silver-mediated direct trifluoromethoxylation of α-diazo esters and ketosteroid was disclosed. The reactions of alkyl α-diazo arylacetates with AgOCF3 or CF3SO2OCF3/AgF at −30 to 10 °C under a N2 atmosphere provided α-trifluoromethoxyl arylacetates in up to 90% yield, while alkyl α-diazo vinylacetates reacting with CF3SO2OCF3/AgF or AgOCF3 afforded γ-trifluoromethoxyl α,β-unsaturated esters in up to 94% yield. The α-diazo ketosteroid was also trifluoromethoxylated under the standard reaction conditions. This protocol allows for an effective and convenient access to a large number of synthetic building blocks, which are promising in the development of new functional OCF3-molecules.
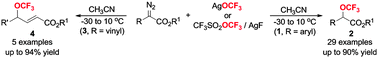

 Please wait while we load your content...
Please wait while we load your content...