Enantioselective construction of imidazolines having vicinal tetra-substituted stereocenters by direct Mannich reaction of α-substituted α-isocyanoacetates with ketimines†
Abstract
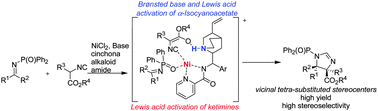
The catalytic Mannich reaction of α-substituted α-isocyanoacetates with ketimines has been investigated. A variety of imidazolines containing congested vicinal tetra-substituted stereocenters were obtained in excellent yields with high diastereo- and enantioselectivity using cinchona alkaloid amide/Ni(II) and base catalysts.

 Please wait while we load your content...
Please wait while we load your content...