Samarocene oxide: from an undesired decomposition product to a new reagent†
Abstract
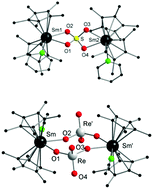
Samarocene oxide [Cp*2Sm-O-SmCp*2] is mostly considered as an undesired decomposition product of the well-established but highly air-sensitive samarocene, [Cp*2Sm(thf)2]. [Cp*2Sm-O-SmCp*2] is often formed by accidental exposure of [Cp*2Sm(thf)2] to air or wet solvents. We show here that [Cp*2Sm-O-SmCp*2] acts as a mild oxide base and thus is a valuable synthetic equivalent for “O2−”. The reaction of [Cp*2Sm-O-SmCp*2] with the inorganic and organic anhydrides COS, CS2, SO2, SO3, Re2O7, and (PhC(O))2O at room temperature resulted in each case in an O2− insertion. The corresponding products were isolated as their samarium complexes.

 Please wait while we load your content...
Please wait while we load your content...