Radical perfluoroalkylation – easy access to 2-perfluoroalkylindol-3-imines via electron catalysis†
Abstract
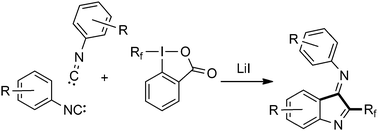
Arylisonitriles (2 equivalents) react with alkyl and perfluoroalkyl radicals to form 2-alkylated indole-3-imines via two sequential additions to the isonitrile moiety followed by homolytic aromatic substitution. The three component reaction comprises three C–C bond formations. The endocyclic imine functionality in the products is more reactive in follow up chemistry and hydrolysis of the exocyclic imine leads to 3-oxindoles that show fluorescence properties.

 Please wait while we load your content...
Please wait while we load your content...