Operationally simple hydrotrifluoromethylation of alkenes with sodium triflinate enabled by Ir photoredox catalysis†
Abstract
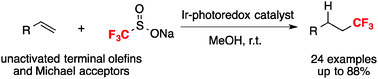
We report herein a single component Ir photoredox catalyst which is capable of catalyzing the hydrotrifluoromethylation of terminal alkenes and Michael acceptors with sodium triflinate (Langlois reagent) in methanol under irradiation at room temperature. Various synthetically useful functional groups, including ester, amide, ether, aldehyde, sulfone, ketone and aryl boronate, are well tolerated in this reaction.

 Please wait while we load your content...
Please wait while we load your content...