Auto-oxidative hydroxysulfenylation of alkenes†
Abstract
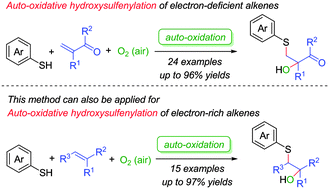
One-pot auto-oxidation mediated hydroxysulfenylation of electron-deficient and electron-rich olefins with phenthiols was explored. The method illustrates a selective and convenient synthesis of complex β-hydroxysulfides using O2 as both the oxidant and the oxygen source under mild transition-metal-free conditions. The application of this new methodology to the gram-scale synthesis of anti-cancer drug bicalutamide has been accomplished in a two-step sequence with 71% overall yield. A plausible radical involved mechanism is proposed.

 Please wait while we load your content...
Please wait while we load your content...