Palladium-catalyzed directing group-assisted C8-triflation of naphthalenes†
Abstract
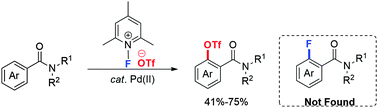
The transition-metal-catalyzed direct triflation of naphthyl amides and naphthyl ketones has been accomplished for the first time. Benzophenone (BP) was found to be a suitable ligand for the cross-coupling reactions. Density functional theory (DFT) calculations revealed that excessive amounts of HOTf inhibit the reductive elimination of the C–F bond to realize the unusual reductive elimination of the C–OTf bond.

 Please wait while we load your content...
Please wait while we load your content...