Copper-catalyzed regio- and enantioselective aminoboration of alkylidenecyclopropanes: the synthesis of cyclopropane-containing β-aminoalkylboranes†
Abstract
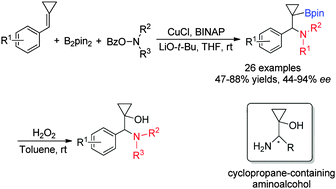
A Cu-catalyzed regio- and enantioselective aminoboration of alkylidenecyclopropanes (ACPs) with bis(pinacolato)-diboron (B2pin2) and hydroxylamines has been described in this paper, affording the corresponding cyclopropane-containing β-aminoalkylboranes in good yields under mild conditions. Moreover, a catalytic asymmetric variant of this transformation has also been realized by using a copper complex with a (S)-BINAP ligand along with further transformation of the product to give cyclopropane-containing 1,2-aminoalcohols.

 Please wait while we load your content...
Please wait while we load your content...