Synthesis of highly substituted γ-hydroxybutenolides through the annulation of keto acids with alkynes and subsequent hydroxyl transposition†
Abstract
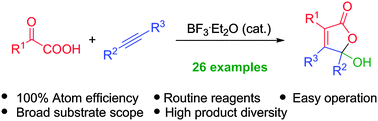
An efficient synthesis of highly functionalized γ-hydroxybutenolides through BF3-catalyzed annulation of keto acids with alkynes is described. Many advantages such as the use of routine reagents, easy operation, and a 100% atom efficiency are demonstrated in the method. The reaction can be readily scaled up to gram quantities, offering good practicality.

 Please wait while we load your content...
Please wait while we load your content...