Spectroscopic and kinetic evidence for redox cycling, catalase and degradation activities of MnIII(TPPS) in a basic aqueous peroxide medium†
Abstract
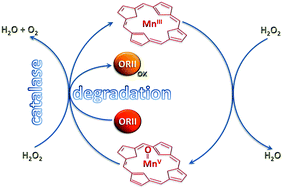
MnIII(TPPS) was found to react rapidly with hydrogen peroxide in basic aqueous solution to form intermediate (TPPS)MnV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and (TPPS)MnIV
O and (TPPS)MnIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species which, in the presence of excess H2O2, are reduced fully back to MnIII(TPPS) with clear evidence for redox cycling of MnIII(TPPS). The system shows very strong catalase and degradation activities.
O species which, in the presence of excess H2O2, are reduced fully back to MnIII(TPPS) with clear evidence for redox cycling of MnIII(TPPS). The system shows very strong catalase and degradation activities.

 Please wait while we load your content...
Please wait while we load your content...