Bench-stable frustrated Lewis pair chemistry: fluoroborate salts as precatalysts for the C–H borylation of heteroarenes†
Abstract
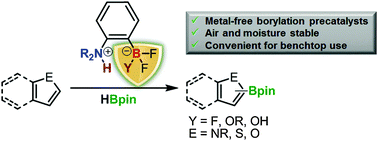
While the organotrifluoroborate group is commonly used as a leaving group in cross-coupling reactions, we now show that their high stability can be used to protect the Lewis acidic moieties of frustrated Lewis pair catalysts. Indeed, the air and moisture-stable trifluoro- and difluoroborate derivatives of bulky (tetramethylpiperidino)benzene are shown to be conveniently converted to their dihydroborane analogue which is known to activate small molecules. An efficient synthesis route to these stable and convenient precatalysts, their deprotection chemistry and their benchtop use for the dehydrogenative borylation of heteroarenes is presented.

 Please wait while we load your content...
Please wait while we load your content...