Chiral-at-metal iridium complex for efficient enantioselective transfer hydrogenation of ketones†
Abstract
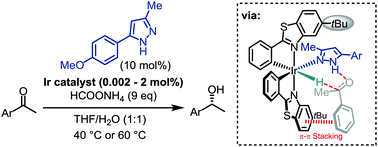
A bis-cyclometalated iridium(III) complex with metal-centered chirality catalyzes the enantioselective transfer hydrogenation of ketones with high enantioselectivities at low catalyst loadings down to 0.002 mol%. Importantly, the rate of catalysis and enantioselectivity are markedly improved in the presence of a pyrazole co-ligand. The reaction is proposed to proceed via an iridium-hydride intermediate exploiting metal–ligand cooperativity (bifunctional catalysis).

 Please wait while we load your content...
Please wait while we load your content...