Retention of chirality in electron-induced reactions†
Abstract
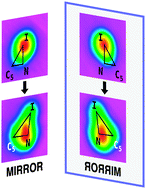
Two enantiomers were observed by Scanning Tunneling Microscopy (STM) when meta-iodopyridine was physisorbed on a 4.6 K Cu(110) surface. The chirality of the reagent was retained in the products of the electron-induced reaction. Dynamical calculations showed this to be a consequence of the reaction occurring on one side of the mirror plane.

 Please wait while we load your content...
Please wait while we load your content...