Discovery of a pyruvylated peptide-metabolizing enzyme using a fluorescent substrate-based protein discovery technique†
Abstract
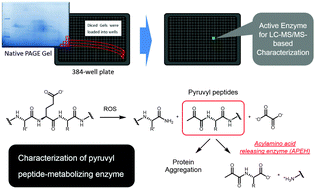
We employed a fluorescent substrate-based target discovery approach to screen the enzymome for metabolic activity towards pyruvyl-amidated peptides, and identified an acylamino acid-releasing enzyme (APEH). Cells overexpressing APEH exhibited higher metabolic activity towards the probe, N-pyruvyl-leucyl-7-amido-4-methylcoumarin (Pyr-Leu-AMC), while the selective APEH inhibitor AA74-1 blocked the reaction. Metabolism of various pyruvylated peptides in liver lysate was almost completely blocked by AA74-1. Pyruvyl peptides are synthesized in response to oxidative stress, but their biological role is poorly understood; identification of a key contributor to their metabolism should stimulate research on pathways leading from oxidative stress to protein modification and biological output.

 Please wait while we load your content...
Please wait while we load your content...