Regioselective iodoamination of terminal ynamides for the synthesis of α-amino-β,β-diiodo-enamides†
Abstract
A mild and efficient methodology involving the I2/TBHP-mediated intermolecular iodoamination of ynamides with amines for the synthesis of α-amino-β,β-diiodo-enamides was developed. This reaction provides the first intermolecular iodoamination of terminal alkynes to diiodo-enamines and can be easily applied to a wide range of substrates.
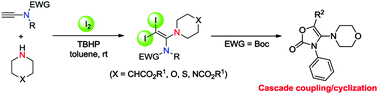

 Please wait while we load your content...
Please wait while we load your content...