Organic base-catalysed solvent-tuned chemoselective carbotrifluoromethylation and oxytrifluoromethylation of unactivated alkenes†
Abstract
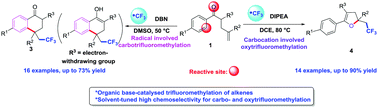
An unprecedented and efficient organic base-catalysed highly chemoselective carbo- and oxytrifluoromethylation of unactivated alkenes with Togni's reagent was developed. The switchable chemoselectivity was tuned by simply changing the organic base catalyst and solvent. Mechanistic studies indicated that a radical cyclization pathway for carbotrifluoromethylation in DMSO and a carbocation pathway for oxytrifluoromethylation in DCE were probably involved.
- This article is part of the themed collection: 2016 Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...