Palladium-catalyzed cross-coupling of enamides with sterically hindered α-bromocarbonyls†
Abstract
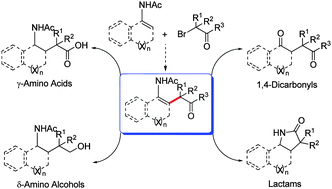
Palladium-catalyzed intermolecular alkylation of enamides with α-bromo carbonyls was developed. Under mild reaction conditions, various cyclic and acyclic enamides reacted well with α-bromo carbonyls to afford the corresponding multi-substituted alkene products in good yields. The coupling products could be converted into very useful γ-amino acid, δ-amino alcohol, 1,4-dicarbonyl and γ-lactam derivatives.

 Please wait while we load your content...
Please wait while we load your content...