Aerobic oxidative cyclization of benzamides via meta-selective C–H tert-alkylation: rapid entry to 7-alkylated isoquinolinediones†
Abstract
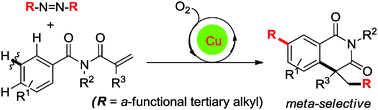
A novel copper-catalyzed aerobic oxidative cyclization of benzamides via meta-selective C–H tert-alkylation using AIBN and analogues as radical precursors was described. This strategy provides an elusive and rapid means to 7-tert-alkylated isoquinolinediones, as well as the construction of tertiary alkyl–aryl C(sp3)–C(sp2) bonds with positional selectivity.

 Please wait while we load your content...
Please wait while we load your content...