Brønsted acid-catalysed conjugate addition of photochemically generated α-amino radicals to alkenylpyridines†
Abstract
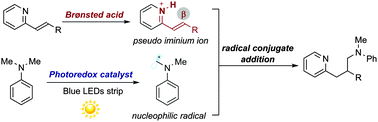
The conjugate addition of α-amino radicals to alkenylpyridines has been accomplished by the synergistic merger of Brønsted acid and visible light photoredox catalysis. Key to reaction development was the protonation of the alkenylpyridines that transiently generated a highly reactive, electrophilic pseudo-iminium ion intermediate. Initial investigations using chiral phosphoric acids provide clues on the feasibility of an enantioselective catalytic variant.

 Please wait while we load your content...
Please wait while we load your content...