Metal assisted cyclomerization of benzodipyrrins into expanded norroles, aza-heptalene and acyclic dimers†
Abstract
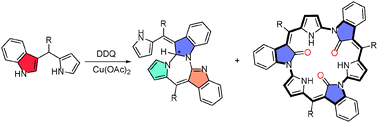
Benzofused dipyrrins react with metal salt copper(II) acetate to predominantly yield a cyclodimer along with a cyclotrimer. N-confused monobenzo-dipyrrin cyclomerized to trioxo-expanded norrole 13a and an acyclic dimer 14 whereas doubly N-confused monobenzo and dibenzo-dipyrrins yielded aza-heptalene 15 and an acyclic dimer 16.

 Please wait while we load your content...
Please wait while we load your content...