Sequential Au(i)/chiral tertiary amine catalysis: a tandem C–H functionalization of anisoles or a thiophene/asymmetric Michael addition sequence to quaternary oxindoles†
Abstract
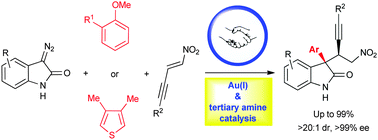
We report an unprecedented sequential Au(I)/bifunctional tertiary amine catalysis, which enables a tandem C–H functionalization of weak nucleophiles (anisoles or thiophenes) and asymmetric Michael addition for the highly enantioselective synthesis of quaternary oxindoles from diazooxindoles and nitroenynes.

 Please wait while we load your content...
Please wait while we load your content...