Asymmetric synthesis of 3,3′-pyrrolidinyl-dispirooxindoles via a one-pot organocatalytic Mannich/deprotection/aza-Michael sequence†
Abstract
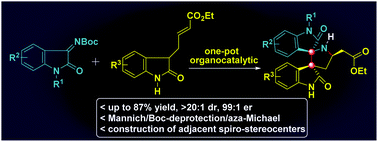
A highly stereoselective synthesis of functionalized 3,3′-pyrrolidinyl-dispirooxindole derivatives with three stereogenic centers, including two contiguous spiro-stereocenters, has been achieved through an organocatalytic Mannich/Boc-deprotection/aza-Michael sequence. Employing the commercially available (DHQD)2PHAL as the catalyst, the scalable reaction occurs with good yields and excellent stereoselectivities, providing a short entry into a series of 3,3′-pyrrolidinyl-dispirooxindoles of potentially medical value.

 Please wait while we load your content...
Please wait while we load your content...