Stereoselective synthesis of 1,3-disubstituted isoindolines via Rh(iii)-catalyzed tandem oxidative olefination–cyclization of 4-aryl cyclic sulfamidates†
Abstract
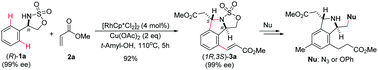
Rh(III)-catalyzed tandem ortho C–H olefination of cyclic 4-aryl sulfamidates (1) and subsequent intramolecular cyclization are described. This reaction serves as a method for the direct and stereoselective synthesis of 1,3-disubstituted isoindolines (3) starting with enantiomerically enriched 4-aryl cyclic sulfamidates. In this process, the configurational integrity of the stereogenic center in the starting cyclic sulfamidate is completely retained. In addition, the process generates trans-1,3-disubstituted isoindolines exclusively.


 Please wait while we load your content...
Please wait while we load your content...