Betaine mediated synthesis of annulated dihydrofurans from oxobis(methylthio)ketene acetals and N-butyl-N′-methyl ethane-1,2-diamine as precursors via NHC elimination†
Abstract
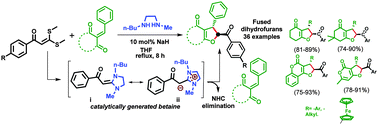
First in situ generation of a betaine intermediate has been developed using two new precursors oxobis(methylthio)ketene acetals and N-butyl-N′-methyl ethane-1,2-diamine for the synthesis of annulated dihydrofurans. This protocol adds a new dimension for the formation of annulated dihydrofurans through a series of selective consecutive formation of C–C and C–O bonds after reacting with enone rings. This in situ generated betaine intermediate corresponds to deoxy-Breslow intermediates in the reaction via elimination of NHC.

 Please wait while we load your content...
Please wait while we load your content...