Sequential meta-C–H olefination of synthetically versatile benzyl silanes: effective synthesis of meta-olefinated toluene, benzaldehyde and benzyl alcohols†
Abstract
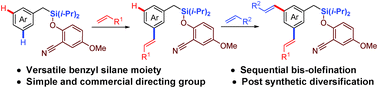
Tremendous progress has been made towards ortho-selective C–H functionalization in the last three decades. However, the activation of distal C–H bonds and their functionalization has remained fairly underdeveloped. Herein, we report sequential meta-C–H functionalization by performing selective mono-olefination and bis-olefination with late stage modification of the C–Si as well as Si–O bonds. Temporary silyl connection was found to be advantageous due to its easy installation, easy removal and wide synthetic diversification.

 Please wait while we load your content...
Please wait while we load your content...