A naphthalimide-based fluorescent sensor for halogenated solvents†
Abstract
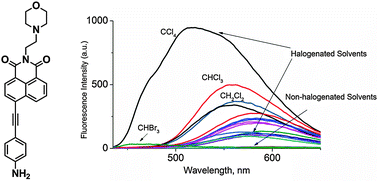
A fluorescent sensor for halogenated solvents termed AMN is reported. AMN shows strong fluorescence in most halogenated solvents (QE > 0.1) but weak fluorescence (QE<0.01) in most non-halogenated solvents. In chlorinated solvents, the fluorescence intensity decreased with the reduction of chlorine content. On the contrary, in brominated solvents the fluorescence intensity increased with the reduction of bromine content. It is worth mentioning that AMN displayed fluorescence emission centered at 520 nm in CCl4 with a quantum yield of 0.607, at 556 nm in CHCl3 with a quantum yield of 0.318, at 584 nm in CH2Cl2 with a quantum yield of 0.128, whereas in CHBr3 was centered at 441 nm with a quantum yield of 0.012. AMN was shown to have the ability to differentiate CCl4, CHCl3, CH2Cl2 and CHBr3 halogenated solvents.

 Please wait while we load your content...
Please wait while we load your content...