Synthesis of inherently chiral calixarenes via direct mercuration of the partial cone conformation†
Abstract
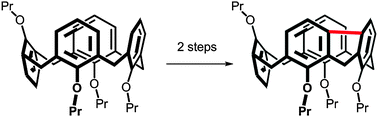
Direct mercuration of calix[4]arene immobilized in the partial cone conformation led to the meta-substituted isomer which was subsequently subjected to Pd-catalysed coupling (C–H activation) with the neighbouring aromatic subunit. Regioselective mercuration thus enabled access to a novel type of inherently chiral calixarenes with a highly distorted cavity potentially applicable to the design of new chiral receptors.

 Please wait while we load your content...
Please wait while we load your content...