Ester-directed Ru-catalyzed C–O activation/C–C coupling reaction of ortho-methoxy naphthoates with organoboroneopentylates†
Abstract
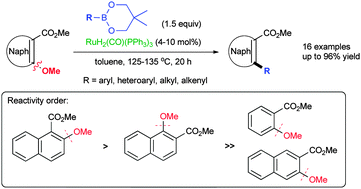
A new, catalytic and general synthetic methodology for the construction of biaryls and heterobiaryls by the cross-coupling of ortho-methoxy naphthoates with organoboroneopentylates is disclosed. The reaction proceeds under RuH2(CO)(PPh3)3-catalyzed conditions driven by unreactive C–O bond activation of a proximate ester directing group (DG)-catalyst chelation. This one-step synthesis of 2-aryl and -heteroaryl-1-naphthoates has the features of operational simplicity, minimum waste and convenient scale-up. The hierarchy of C(O)Me > CONEt2 > CO2Me coordination-assisted reactivity, of potential value in chemoselective synthesis, is also established.

 Please wait while we load your content...
Please wait while we load your content...