Chiral phosphoric acid catalyzed enantioselective 1,3-dipolar cycloaddition reaction of azlactones†
Abstract
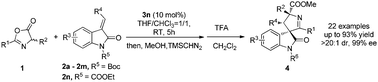
The first chiral phosphoric acid catalyzed highly diastereo- and enantioselective 1,3-dipolar cycloaddition reaction of azlactones and methyleneindolinones was disclosed. By using a BINOL-derived chiral phosphoric acid as the catalyst, azlactones were activated as chiral anti N-protonated 1,3-dipoles to react with methyleneindolinones to yield biologically important 3,3′-pyrrolidonyl spirooxindole scaffolds in high yields, with good-to-excellent diastereo- and enantioselectivity.

 Please wait while we load your content...
Please wait while we load your content...