Synthesis of the aglycon of aspafiliosides E and F based on cascade reactions†
Abstract
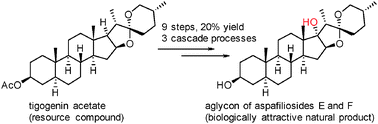
A synthesis of C17α-OH-tigogenin, the aglycon of aspafiliosides E and F, is described. The main features of the synthesis are three cascade processes, which involve the iodo-lactonization of furostan-26-acid to open ring E, a cascade hydrolysis/intramolecular SN2 process to close ring E, and a cascade intramolecular redox-ketalization process to close ring F. This synthesis would enrich the strategies used for the manipulation of spiroketals in steroidal sapogenins and other substrates.


 Please wait while we load your content...
Please wait while we load your content...