Metal-free oxidative cyclization of acetophenones with diamines: a facile access to phenylpyridines†‡
Abstract
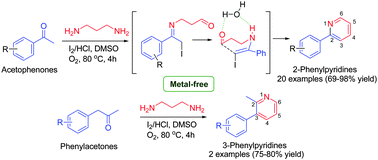
An efficient metal-free access to 2- and 3-phenylpyridines via oxidative coupling of acetophenones or phenylacetones with 1,3-diaminopropane has been described. The reaction involves shorter reaction time, excellent yields and a broad substrate scope. The reaction proceeds via the formation of imine, which further undergoes oxidative C–N bond cleavage, C–C bond formation and oxidation to give a pyridine skeleton. The quantum chemical calculations identified the transition state for the reaction and helped in tracing the reaction mechanism.

 Please wait while we load your content...
Please wait while we load your content...